
› Bringing Proof
of Principle
› Targeting a unique antigenic domain of carcinoembryonic antigen (CEA) is opening the door to a new breed of protein-based vaccines that block metastasis.
Human carcinoembryonic antigen (CEA) has long been known to play an important cell-surface role in adhesion events between cells. It was first identified in 1965 by Canadian scientists Phil Gold & Sam Freedman, and the gene was later cloned in 1990 by Wolfgang Zimmerman’s group in Freiberg, Germany. In reality, CEA is a family of closely related glycoproteins that are characterised by a series of immunoglobulin-like domains, and are tethered to the cell membrane by a glycosyl phosphatidyl inositol (GPI) anchor.
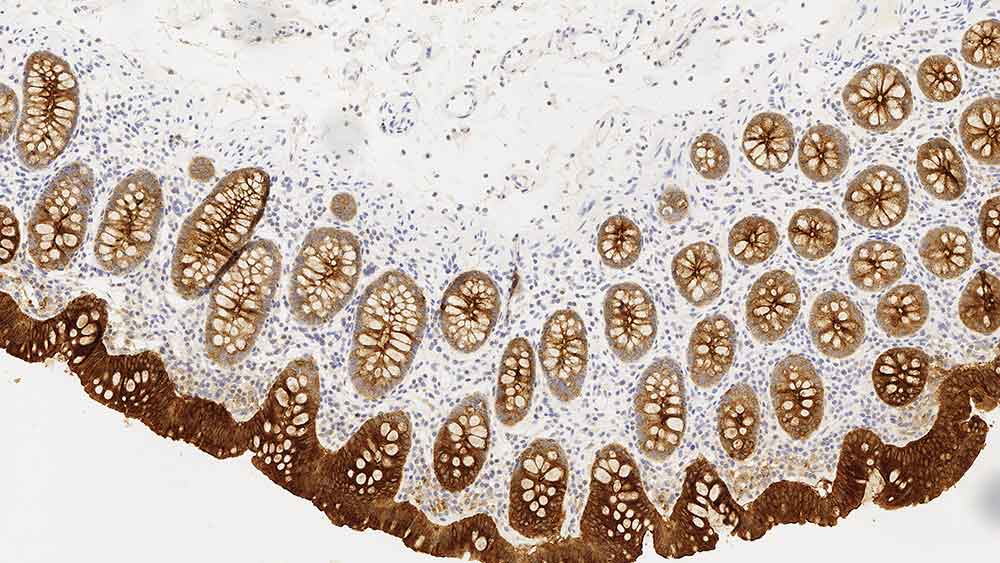
» The image shows immunohistochemical staining of human bowel tissue for CEA. Below is a representation of the CEA polypeptide structure derived from X-ray crystallography and neutron solution scattering.

CEA is well established to be involved in a number of important adhesion events, and have a role to play in the implantation of tumor cells during the process of metastasis, which is a pivotal stage in the progression of cancer. The IgV domain of CEA is involved in both heterotypic and homotypic interactions that are relevant to the formation of micrometastases. D5’s patented MetVax targets this crucial domain.
Priming the immune system to recognise CEA using a protein-based vaccine has the potential to intervene in this pathway, preventing primary tumor cells from spreading effectively and colonizing healthy tissue.
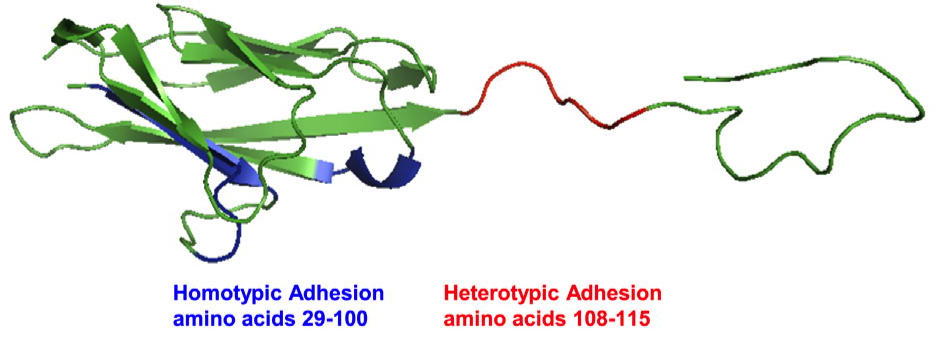
› A ribbon structure of the CEA N domain highlighting the two areas necessary for the engraftment of CEA-expressing tumor cells. Amino acid residues 29-100 (highlighted in blue) are present on the CFCʹCʺG of the CEA N domain and are necessary for homotypic interactions while amino acid residues 108-115 (highlighted in red) are required for the association of the CEA N domain with fibronectin.
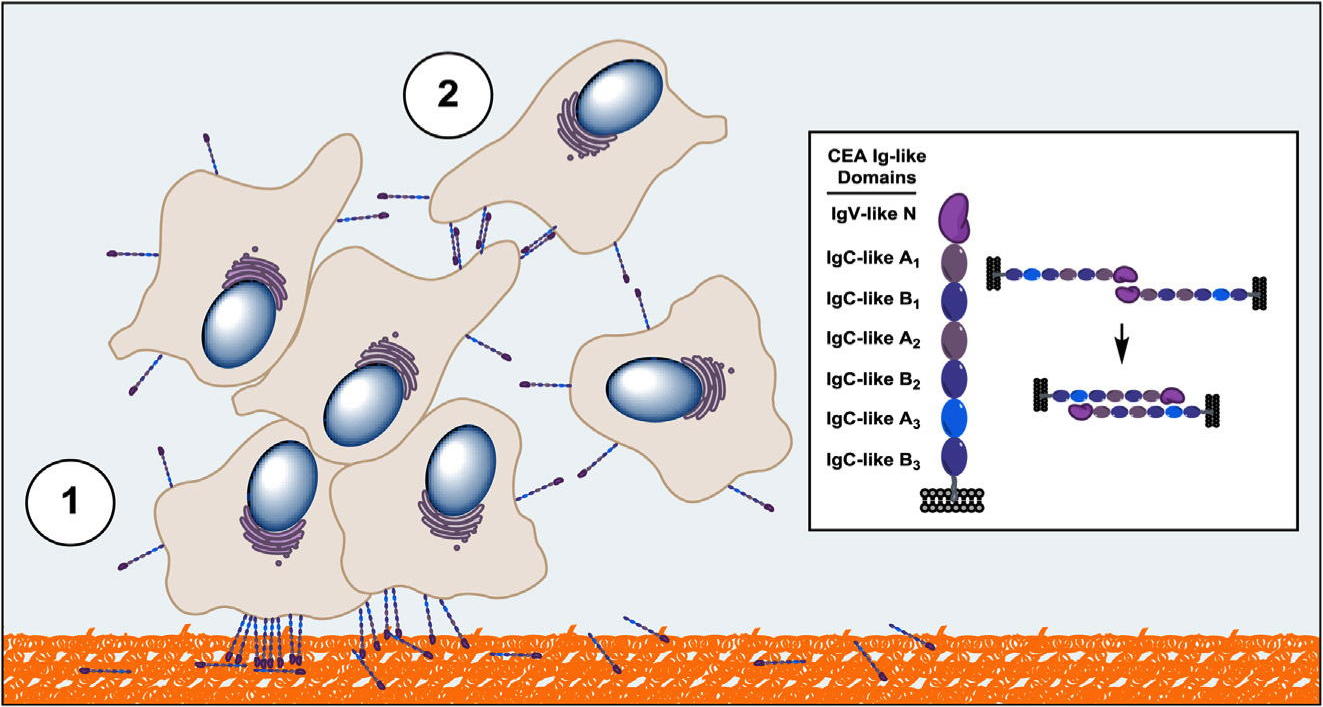
› Schematic illustrating key binding events linking the CEA N domain to CEA-expressing tumor cell implantation and aggregation. In this model, CEA-expressing cells interact via their N domain with proteins of the extracellular matrix such as fibronectin (Step 1, orange-colored matrix), allowing such cells to engraft at a site distal to a primary tumor site. Surviving and proliferating CEA-expressing cancer cells subsequently bind to each other through homotypic interactions involving their CEA N and A3 domains (Step 2). Expanding CEA-expressing cell populations lead to cellular aggregates through multivalent interactions leading to the occurrence of micrometastases.
› Targeting CD200R1 offers a new mechanism for intervention in numerous human immune diseases.
Aptamers that agonize CD200R1 dampen immune responses and thereby have the potential to suppress unwanted inflammation in conditions such as rheumatoid arthritis, Crohn’s disease, psoriasis and other widespread indications. Aptamers that antagonize CD200R1 unshackle immune cell regulation and have value in cancer immunotherapy.
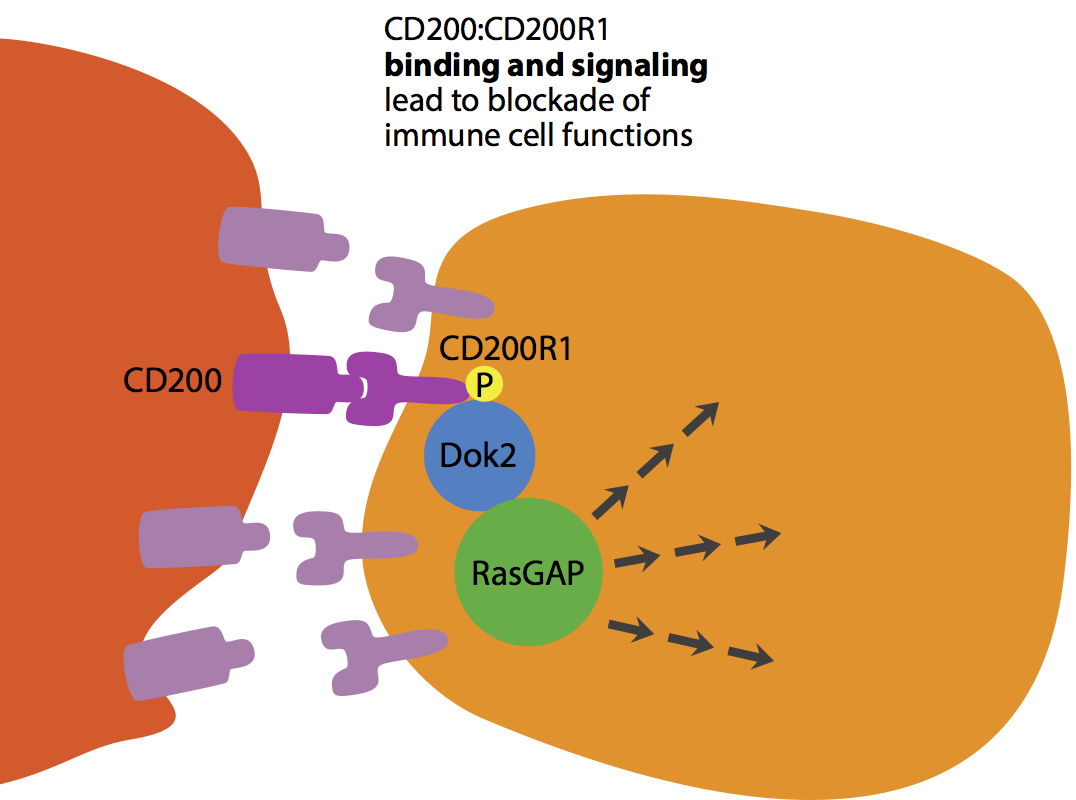
› The figure illustrates the immune modulating interaction between the Type 1 membrane glycoprotein, CD200, and its corresponding receptor, CD200R1. While CD200 is widely expressed on the surface of many cells (including immune cells), its cognate receptor, CD200R1, seems to be restricted to cells of the lymphoid and myeloid lineage. When the receptor is engaged with the ligand, it delivers immune inhibitory signals to modulate inflammatory responses by way of phosphorylation of proteins such as Dok2, which in turn recruits RasGAP (namely Ras GTPase activating protein). This latter protein converts the active GTP-bound family of proteins called Ras into their inactive GDP-bound state by stimulating Ras’ endogenous GTPase activity. While active, the Ras family of proteins serve to modulate a variety of functions, including upregulation of the immune response in inflammatory pathways through molecules such as cytokines.
CD200–CD200R1 signalling has been implicated in a number of immune related diseases such as allergic disorders, arthritis, infection, transplantation, and autoimmune disorders such as systemic lupus erythematosus and multiple sclerosis.
CD200R1 expressed on the surface of myeloid and lymphoid cells delivers immune inhibitory signals to dampen inflammatory responses when agonised by its natural ligand CD200. D5Pharma’s small, synthetic PEGylated DNA aptamer, PEG:CCS13, can serve as a substitute agonist to stimulate this pathway and tune down the immune system in exactly the same manner as much larger, soluble protein analogues such as CD200Fc, the extracellular immunoglobulin-like domain of CD200. In a murine model of transplantation, bolus intravenous injection of this PEGylated aptamer enhanced survival of allogeneic skin grafts as effectively as a soluble CD200Fc.
Read more about our unique approach in these academic publications
The IgV-like N domain of CEA plays a critical role in the implantation of metastatic tumor cells
Our findings point to the importance of a specific region of CEA in cancer progression.
Vaccination with MetVax prevents tumor colonization and the spread of disease.
D5’s scientists have shown that MetVax can actively prevent tumor implantation.
PEG:CCS13 is a potent DNA-based immunosuppressant.
D5Pharma’s most recent publication on PEG:CCS13: Prodeus, et al., Molecular Therapy Nucleic Acids (2018) 12, 350.

